In a
chemical reaction,
chemical equilibrium is the state in which both reactants and products are present in
concentrations which have no further tendency to change with time.
[1] Usually, this state results when the forward reaction proceeds at the same rate as the
reverse reaction. The
reaction rates
of the forward and backward reactions are generally not zero, but
equal. Thus, there are no net changes in the concentrations of the
reactant(s) and product(s). Such a state is known as
dynamic equilibrium.
Historical Introduction
Burette, a common laboratorl apparatus for carrying out titration, an important experimental technique in equilibrium and analytical chemistry.
The concept of chemical equilibrium was developed after
Berthollet (1803) found that some
chemical reactions are
reversible. For any reaction mixture to exist at equilibrium, the
rates of the forward and backward (reverse) reactions are equal. In the following
chemical equation with arrows pointing both ways to indicate equilibrium, A and B are
reactant chemical species, S and T are product species, and
α,
β,
σ, and
τ are the
stoichiometric coefficients of the respective reactants and products:
- α A + β B ⇌ σ S + τ T
The equilibrium concentration position of a reaction is said to lie
"far to the right" if, at equilibrium, nearly all the reactants are
consumed. Conversely the equilibrium position is said to be "far to the
left" if hardly any product is formed from the reactants.
Guldberg and
Waage (1865), building on Berthollet’s ideas, proposed the
law of mass action:


where A, B, S and T are
active masses and
k+ and
k− are
rate constants. Since at equilibrium forward and backward rates are equal:

and the ratio of the rate constants is also a constant, now known as an
equilibrium constant.

By convention the products form the
numerator. However, the
law of mass action is valid only for concerted one-step reactions that proceed through a single
transition state and is
not valid in general because
rate equations do not, in general, follow the
stoichiometry of the reaction as Guldberg and Waage had proposed (see, for example,
nucleophilic aliphatic substitution by S
N1 or reaction of
hydrogen and
bromine to form
hydrogen bromide). Equality of forward and backward reaction rates, however, is a
necessary condition for chemical equilibrium, though it is not
sufficient to explain why equilibrium occurs.
Despite the failure of this derivation, the equilibrium constant for a
reaction is indeed a constant, independent of the activities of the
various species involved, though it does depend on temperature as
observed by the
van 't Hoff equation. Adding a
catalyst
will affect both the forward reaction and the reverse reaction in the
same way and will not have an effect on the equilibrium constant. The
catalyst will speed up both reactions thereby increasing the speed at
which equilibrium is reached.
[2][4]
Although the
macroscopic equilibrium concentrations are constant in time, reactions do occur at the molecular level. For example, in the case of
acetic acid dissolved in water and forming
acetate and
hydronium ions,
- CH3CO2H + H2O ⇌ CH
3CO−
2 + H3O+
a proton may hop from one molecule of acetic acid on to a water
molecule and then on to an acetate anion to form another molecule of
acetic acid and leaving the number of acetic acid molecules unchanged.
This is an example of
dynamic equilibrium. Equilibria, like the rest of thermodynamics, are statistical phenomena, averages of microscopic behavior.
Le Châtelier's principle (1884) gives an idea of the behavior of an equilibrium system when changes to its reaction conditions occur.
If
a dynamic equilibrium is disturbed by changing the conditions, the
position of equilibrium moves to partially reverse the change. For
example, adding more S from the outside will cause an excess of
products, and the system will try to counteract this by increasing the
reverse reaction and pushing the equilibrium point backward (though the
equilibrium constant will stay the same).
If
mineral acid
is added to the acetic acid mixture, increasing the concentration of
hydronium ion, the amount of dissociation must decrease as the reaction
is driven to the left in accordance with this principle. This can also
be deduced from the equilibrium constant expression for the reaction:

If {H
3O
+} increases {CH
3CO
2H} must increase and
CH
3CO−
2 must decrease. The H
2O is left out, as it is the solvent and its concentration remains high and nearly constant.
A quantitative version is given by the
reaction quotient.
J. W. Gibbs suggested in 1873 that equilibrium is attained when the
Gibbs free energy
of the system is at its minimum value (assuming the reaction is carried
out at constant temperature and pressure). What this means is that the
derivative of the Gibbs energy with respect to
reaction coordinate (a measure of the
extent of reaction that has occurred, ranging from zero for all reactants to a maximum for all products) vanishes, signalling a
stationary point. This derivative is called the reaction Gibbs energy (or energy change) and corresponds to the difference between the
chemical potentials of reactants and products at the composition of the reaction mixture.
[1]
This criterion is both necessary and sufficient. If a mixture is not at
equilibrium, the liberation of the excess Gibbs energy (or
Helmholtz energy
at constant volume reactions) is the "driving force" for the
composition of the mixture to change until equilibrium is reached. The
equilibrium constant can be related to the standard
Gibbs free energy change for the reaction by the equation

where R is the
universal gas constant and T the
temperature.
When the reactants are
dissolved in a medium of high
ionic strength the quotient of
activity coefficients may be taken to be constant. In that case the
concentration quotient,
Kc,
![{\displaystyle K_{\mathrm {c} }={\frac {[\mathrm {S} ]^{\sigma }[\mathrm {T} ]^{\tau }}{[\mathrm {A} ]^{\alpha }[\mathrm {B} ]^{\beta }}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1343be135c4f33c7333e53558b9e96d27f1819ed)
where [A] is the
concentration of A, etc., is independent of the
analytical concentration of the reactants. For this reason, equilibrium constants for
solutions are usually
determined in media of high ionic strength.
Kc varies with
ionic strength, temperature and pressure (or volume). Likewise
Kp for gases depends on
partial pressure. These constants are easier to measure and encountered in high-school chemistry courses.
Thermodynamics
At constant temperature and pressure, one must consider the
Gibbs free energy,
G, while at constant temperature and volume, one must consider the
Helmholtz free energy:
A, for the reaction; and at constant internal energy and volume, one must consider the entropy for the reaction:
S.
The constant volume case is important in
geochemistry and
atmospheric chemistry where pressure variations are significant. Note that, if reactants and products were in
standard state
(completely pure), then there would be no reversibility and no
equilibrium. Indeed, they would necessarily occupy disjoint volumes of
space. The mixing of the products and reactants contributes a large
entropy (known as
entropy of mixing)
to states containing equal mixture of products and reactants. The
standard Gibbs energy change, together with the Gibbs energy of mixing,
determine the equilibrium state.
[5][6]
In this article only the
constant pressure case is considered. The relation between the Gibbs free energy and the equilibrium constant can be found by considering
chemical potentials.
[1]
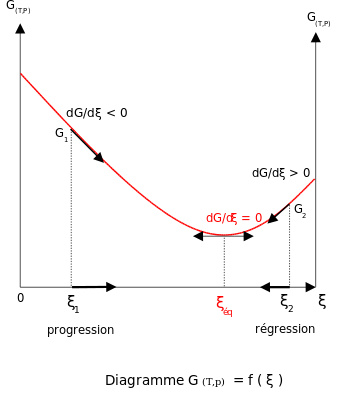
At constant temperature and pressure, the
Gibbs free energy,
G, for the reaction depends only on the
extent of reaction:
ξ (Greek letter
xi), and can only decrease according to the
second law of thermodynamics. It means that the derivative of
G with
ξ must be negative if the reaction happens; at the equilibrium the derivative being equal to zero.
 : equilibrium
: equilibrium
In order to meet the thermodynamic condition for equilibrium, the
Gibbs energy must be stationary, meaning that the derivative of
G with respect to the
extent of reaction:
ξ, must be zero. It can be shown that in this case, the sum of
chemical potentials
of the products is equal to the sum of those corresponding to the
reactants. Therefore, the sum of the Gibbs energies of the reactants
must be the equal to the sum of the Gibbs energies of the products.

where
μ is in this case a partial molar Gibbs energy, a
chemical potential. The chemical potential of a reagent A is a function of the
activity, {A} of that reagent.

(where
μo
A is the
standard chemical potential).
The definition of the
Gibbs energy equation interacts with the
fundamental thermodynamic relation to produce
 .
.
Inserting
dNi =
νi dξ into the above equation gives a
Stoichiometric coefficient (

) and a differential that denotes the reaction occurring once (
dξ). At constant pressure and temperature the above equations can be written as
 which is the "Gibbs free energy change for the reaction .
which is the "Gibbs free energy change for the reaction .
This results in:
 .
.
By substituting the chemical potentials:
 ,
,
the relationship becomes:

 :
:
which is the
standard Gibbs energy change for the reaction that can be calculated using thermodynamical tables. The
reaction quotient is defined as:

Therefore,

At equilibrium:

leading to:

and

Obtaining the value of the standard Gibbs energy change, allows the calculation of the equilibrium constant.
Addition of reactants or products
For a reactional system at equilibrium:
Qr =
Keq;
ξ =
ξeq.
- If are modified activities of constituents, the value of the
reaction quotient changes and becomes different from the equilibrium
constant: Qr ≠ Keq
-

- and
-

- then
-

- If activity of a reagent i increases
-
 , the reaction quotient decreases.
, the reaction quotient decreases.
- then
-
 and
and 
- The reaction will shift to the right (i.e. in the forward direction, and thus more products will form).
- If activity of a product j increases
- then
-
 and
and 
- The reaction will shift to the left (i.e. in the reverse direction, and thus less products will form).
Note that activities and equilibrium constants are dimensionless numbers.
Treatment of activity
The expression for the equilibrium constant can be rewritten as the product of a concentration quotient,
Kc and an
activity coefficient quotient,
Γ.
![{\displaystyle K={\frac {[\mathrm {S} ]^{\sigma }[\mathrm {T} ]^{\tau }...}{[\mathrm {A} ]^{\alpha }[\mathrm {B} ]^{\beta }...}}\times {\frac {{\gamma _{\mathrm {S} }}^{\sigma }{\gamma _{\mathrm {T} }}^{\tau }...}{{\gamma _{\mathrm {A} }}^{\alpha }{\gamma _{\mathrm {B} }}^{\beta }...}}=K_{\mathrm {c} }\Gamma }](https://wikimedia.org/api/rest_v1/media/math/render/svg/160fbc88403a037161d2c9a44d10cd44746215e8)
[A] is the concentration of reagent A, etc. It is possible in
principle to obtain values of the activity coefficients, γ. For
solutions, equations such as the
Debye–Hückel equation or extensions such as
Davies equation[7] Specific ion interaction theory or
Pitzer equations[8] may be used.
Software (below). However this is not always possible. It is common practice to assume that
Γ
is a constant, and to use the concentration quotient in place of the
thermodynamic equilibrium constant. It is also general practice to use
the term
equilibrium constant instead of the more accurate
concentration quotient. This practice will be followed here.
For reactions in the gas phase
partial pressure is used in place of concentration and
fugacity coefficient in place of activity coefficient. In the real world, for example, when making
ammonia in industry, fugacity coefficients must be taken into account. Fugacity,
f, is the product of partial pressure and fugacity coefficient. The chemical potential of a species in the gas phase is given by

so the general expression defining an equilibrium constant is valid for both solution and gas phases.
Concentration quotients
In aqueous solution, equilibrium constants are usually determined in the presence of an "inert" electrolyte such as
sodium nitrate NaNO
3 or
potassium perchlorate KClO
4. The
ionic strength of a solution is given by

where
ci and
zi stand for the concentration and ionic charge of ion type
i, and the sum is taken over all the
N
types of charged species in solution. When the concentration of
dissolved salt is much higher than the analytical concentrations of the
reagents, the ions originating from the dissolved salt determine the
ionic strength, and the ionic strength is effectively constant. Since
activity coefficients depend on ionic strength the activity coefficients
of the species are effectively independent of concentration. Thus, the
assumption that
Γ is constant is justified. The concentration quotient is a simple multiple of the equilibrium constant.
[9]

However,
Kc will vary with ionic strength. If it is
measured at a series of different ionic strengths the value can be
extrapolated to zero ionic strength.
[8] The concentration quotient obtained in this manner is known, paradoxically, as a thermodynamic equilibrium constant.
To use a published value of an equilibrium constant in conditions of
ionic strength different from the conditions used in its determination,
the value should be adjusted.
Metastable mixtures
A mixture may appear to have no tendency to change, though it is not at equilibrium. For example, a mixture of
SO2 and
O2 is
metastable as there is a
kinetic barrier to formation of the product,
SO3.
- 2 SO2 + O2 ⇌ 2 SO3
The barrier can be overcome when a
catalyst is also present in the mixture as in the
contact process, but the catalyst does not affect the equilibrium concentrations.
Likewise, the formation of
bicarbonate from
carbon dioxide and
water is very slow under normal conditions
- CO2 + 2 H2O ⇌ HCO−
3 + H3O+
but almost instantaneous in the presence of the catalytic
enzyme carbonic anhydrase.
Pure substances
When pure substances (liquids or solids) are involved in equilibria their activities do not appear in the equilibrium constant
[10] because their numerical values are considered one.
Applying the general formula for an equilibrium constant to the
specific case of a dilute solution of acetic acid in water one obtains
- CH3CO2H + H2O ⇌ CH3CO2− + H3O+
![{\displaystyle K_{\mathrm {c} }={\frac {\mathrm {[{CH_{3}CO_{2}}^{-}][{H_{3}O}^{+}]} }{\mathrm {[{CH_{3}CO_{2}H}][{H_{2}O}]} }}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/53b93bf6dae8354f02ab3e935fca63b9bd4a16c4)
For all but very concentrated solutions, the water can be considered a
"pure" liquid, and therefore it has an activity of one. The equilibrium
constant expression is therefore usually written as
![{\displaystyle K={\frac {\mathrm {[{CH_{3}CO_{2}}^{-}][{H_{3}O}^{+}]} }{\mathrm {[{CH_{3}CO_{2}H}]} }}=K_{\mathrm {c} }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/472d349477dcf83e95cb3c3423e8ba247ff1d23a) .
.
A particular case is the
self-ionization of water itself
- 2 H2O ⇌ H3O+ + OH−
Because water is the solvent, and has an activity of one, the self-ionization constant of water is defined as
![{\displaystyle K_{\mathrm {w} }=\mathrm {[H^{+}][OH^{-}]} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/02e72380cadad70b38e1a6f42ce8b217618bc11f)
It is perfectly legitimate to write [H
+] for the
hydronium ion concentration, since the state of
solvation of the proton is constant (in dilute solutions) and so does not affect the equilibrium concentrations.
Kw varies with variation in ionic strength and/or temperature.
The concentrations of H
+ and OH
− are not independent quantities. Most commonly [OH
−] is replaced by
Kw[H
+]
−1 in equilibrium constant expressions which would otherwise include
hydroxide ion.
Solids also do not appear in the equilibrium constant expression, if
they are considered to be pure and thus their activities taken to be
one. An example is the
Boudouard reaction:
[10]
- 2 CO ⇌ CO2 + C
for which the equation (without solid carbon) is written as:
![{\displaystyle K_{\mathrm {c} }={\frac {\mathrm {[CO_{2}]} }{\mathrm {[CO]^{2}} }}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/df7ff1e7f45707791cfd9dd8e697fe5695e94f60)
Multiple equilibria
Consider the case of a dibasic acid H
2A. When dissolved in water, the mixture will contain H
2A, HA
− and A
2−. This equilibrium can be split into two steps in each of which one proton is liberated.
![{\displaystyle {\begin{array}{rl}{\ce {H2A<=>{HA^{-}}+{H+}}}:&K_{1}={\frac {\ce {[HA-][H+]}}{\ce {[H2A]}}}\\{\ce {HA-<=>{A^{2-}}+{H+}}}:&K_{2}={\frac {\ce {[A^{2-}][H+]}}{\ce {[HA-]}}}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/02fc199cec1e474859edf9723bf1aa772591e5a7)
K1 and
K2 are examples of
stepwise equilibrium constants. The
overall equilibrium constant,
βD, is product of the stepwise constants.
 :
: ![{\displaystyle \beta _{\ce {D}}={\frac {\ce {[A^{2-}][H^{+}]^{2}}}{\ce {[H_{2}A]}}}=K_{1}K_{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/63732493f38cea73de7cc7600a0fdcac4c2250f9)
Note that these constants are
dissociation constants
because the products on the right hand side of the equilibrium
expression are dissociation products. In many systems, it is preferable
to use association constants.
![{\displaystyle {\begin{array}{ll}{\ce {{A^{2-}}+{H+}<=>HA-}}:&\beta _{1}={\frac {\ce {[HA^{-}]}}{\ce {[A^{2-}][H+]}}}\\{\ce {{A^{2-}}+{2H+}<=>H2A}}:&\beta _{2}={\frac {\ce {[H2A]}}{\ce {[A^{2-}][H+]^{2}}}}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3bd8050bd8872f9cbd63bffcc07ebc25c6875c3a)
β1 and
β2 are examples of association constants. Clearly
β1 = 1/K2 and
β2 = 1/βD;
log β1 = pK2 and
log β2 = pK2 + pK1[11] For multiple equilibrium systems, also see: theory of
Response reactions.
Effect of temperature
The effect of changing temperature on an equilibrium constant is given by the
van 't Hoff equation

Thus, for
exothermic reactions (Δ
H is negative),
K decreases with an increase in temperature, but, for
endothermic reactions, (ΔH is positive)
K increases with an increase temperature. An alternative formulation is

At first sight this appears to offer a means of obtaining the
standard molar enthalpy of the reaction by studying the variation of
K
with temperature. In practice, however, the method is unreliable
because error propagation almost always gives very large errors on the
values calculated in this way.
Effect of electric and magnetic fields
The effect of electric field on equilibrium has been studied by
Manfred Eigen among others.
Types of equilibrium
- N2 (g) ⇌ N2 (adsorbed)
- N2 (adsorbed) ⇌ 2 N (adsorbed)
- H2 (g) ⇌ H2 (adsorbed)
- H2 (adsorbed) ⇌ 2 H (adsorbed)
- N (adsorbed) + 3 H(adsorbed) ⇌ NH3 (adsorbed)
- NH3 (adsorbed) ⇌ NH3 (g)
- In the gas phase: rocket engines[12]
- The industrial synthesis such as ammonia in the Haber–Bosch process (depicted right) takes place through a succession of equilibrium steps including adsorption processes
- Atmospheric chemistry
- Seawater and other natural waters: chemical oceanography
- Distribution between two phases
- Acid–base equilibria: acid dissociation constant, hydrolysis, buffer solutions, indicators, acid–base homeostasis
- Metal–ligand complexation: sequestering agents, chelation therapy, MRI contrast reagents, Schlenk equilibrium
- Adduct formation: host–guest chemistry, supramolecular chemistry, molecular recognition, dinitrogen tetroxide
- In certain oscillating reactions, the approach to equilibrium is not asymptotically but in the form of a damped oscillation .[10]
- The related Nernst equation in electrochemistry gives the difference in electrode potential as a function of redox concentrations.
- When molecules on each side of the equilibrium are able to further
react irreversibly in secondary reactions, the final product ratio is
determined according to the Curtin–Hammett principle.
In these applications, terms such as stability constant, formation
constant, binding constant, affinity constant, association/dissociation
constant are used. In biochemistry, it is common to give units for
binding constants, which serve to define the concentration units used
when the constant’s value was determined.
Composition of a mixture
When
the only equilibrium is that of the formation of a 1:1 adduct as the
composition of a mixture, there are any number of ways that the
composition of a mixture can be calculated. For example, see
ICE table for a traditional method of calculating the pH of a solution of a weak acid.
There are three approaches to the general calculation of the composition of a mixture at equilibrium.
- The most basic approach is to manipulate the various equilibrium
constants until the desired concentrations are expressed in terms of
measured equilibrium constants (equivalent to measuring chemical
potentials) and initial conditions.
- Minimize the Gibbs energy of the system.[13][14]
- Satisfy the equation of mass balance.
The equations of mass balance are simply statements that demonstrate
that the total concentration of each reactant must be constant by the
law of conservation of mass.
Mass-balance equations
In general, the calculations are rather complicated or complex. For instance, in the case of a dibasic acid, H
2A dissolved in water the two reactants can be specified as the
conjugate base, A
2−, and the
proton, H
+. The following equations of mass-balance could apply equally well to a base such as
1,2-diaminoethane, in which case the base itself is designated as the reactant A:
![{\displaystyle T_{\mathrm {A} }=\mathrm {[A]+[HA]+[H_{2}A]} \,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b4caf4ff0552bd0430f4b305b3b92c05aef15aa2)
![{\displaystyle T_{\mathrm {H} }=\mathrm {[H]+[HA]+2[H_{2}A]-[OH]} \,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/094ecf5d3e87ba5a02d744835486f24086804cfc)
With T
A the total concentration of species A. Note that it
is customary to omit the ionic charges when writing and using these
equations.
When the equilibrium constants are known and the total concentrations
are specified there are two equations in two unknown "free
concentrations" [A] and [H]. This follows from the fact that [HA] =
β1[A][H], [H
2A] =
β2[A][H]
2 and [OH] =
Kw[H]
−1
![{\displaystyle T_{\mathrm {A} }=\mathrm {[A]} +\beta _{1}\mathrm {[A][H]} +\beta _{2}\mathrm {[A][H]} ^{2}\,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6ae376d767bfb698f8d771a8aa374366c647255)
![{\displaystyle T_{\mathrm {H} }=\mathrm {[H]} +\beta _{1}\mathrm {[A][H]} +2\beta _{2}\mathrm {[A][H]} ^{2}-K_{w}[\mathrm {H} ]^{-1}\,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/25502da2d24e51d7cb6c53782a5392673c4b0819)
so the concentrations of the "complexes" are calculated from the free
concentrations and the equilibrium constants. General expressions
applicable to all systems with two reagents, A and B would be
![{\displaystyle T_{\mathrm {A} }=[\mathrm {A} ]+\sum _{i}p_{i}\beta _{i}[\mathrm {A} ]^{p_{i}}[\mathrm {B} ]^{q_{i}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/de4c20306740d1b3f30387f17ac264b500d313b8)
![{\displaystyle T_{\mathrm {B} }=[\mathrm {B} ]+\sum _{i}q_{i}\beta _{i}[\mathrm {A} ]^{p_{i}}[\mathrm {B} ]^{q_{i}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4c9dea321370bf3d5b01364cfed9dbe3c941ce31)
It is easy to see how this can be extended to three or more reagents.
Polybasic acids
The composition of solutions containing reactants A and H is easy to calculate as a function of
p[H]. When [H] is known, the free concentration [A] is calculated from the mass-balance equation in A.
The diagram alongside, shows an example of the hydrolysis of the
aluminium Lewis acid Al
3+(aq)[15] shows the species concentrations for a 5 × 10
−6 M solution of an aluminium salt as a function of pH. Each concentration is shown as a percentage of the total aluminium.
Solution and precipitation
The diagram above illustrates the point that a
precipitate
that is not one of the main species in the solution equilibrium may be
formed. At pH just below 5.5 the main species present in a 5 μM solution
of Al
3+ are
aluminium hydroxides Al(OH)
2+,
AlOH+
2 and
Al
13(OH)7+
32, but on raising the pH
Al(OH)3 precipitates from the solution. This occurs because Al(OH)
3 has a very large
lattice energy. As the pH rises more and more Al(OH)
3 comes out of solution. This is an example of
Le Châtelier's principle
in action: Increasing the concentration of the hydroxide ion causes
more aluminium hydroxide to precipitate, which removes hydroxide from
the solution. When the hydroxide concentration becomes sufficiently high
the soluble aluminate,
Al(OH)−
4, is formed.
Another common instance where precipitation occurs is when a metal
cation interacts with an anionic ligand to form an electrically neutral
complex. If the complex is
hydrophobic, it will precipitate out of water. This occurs with the
nickel ion Ni
2+ and
dimethylglyoxime, (dmgH
2): in this case the lattice energy of the solid is not particularly large, but it greatly exceeds the energy of
solvation of the molecule Ni(dmgH)
2.
Minimization of Gibbs energy
At equilibrium, at a specified temperature and pressure, the Gibbs energy
G is at a minimum:

For a closed system, no particles may enter or leave, although they
may combine in various ways. The total number of atoms of each element
will remain constant. This means that the minimization above must be
subjected to the constraints:

where
aij is the number of atoms of element
i in molecule
j and
b0
i is the total number of atoms of element
i, which is a constant, since the system is closed. If there are a total of
k types of atoms in the system, then there will be
k such equations. If ions are involved, an additional row is added to the a
ij matrix specifying the respective charge on each molecule which will sum to zero.
This is a standard problem in
optimisation, known as
constrained minimisation. The most common method of solving it is using the method of
Lagrange multipliers, also known as
undetermined multipliers (though other methods may be used).
Define:

where the
λi are the Lagrange multipliers, one for each element. This allows each of the
Nj and
λj to be treated independently, and it can be shown using the tools of
multivariate calculus that the equilibrium condition is given by


(For proof see
Lagrange multipliers.) This is a set of (
m +
k) equations in (
m +
k) unknowns (the
Nj and the
λi) and may, therefore, be solved for the equilibrium concentrations
Nj as long as the chemical potentials are known as functions of the concentrations at the given temperature and pressure. (See
Thermodynamic databases for pure substances.) Note that the second equation is just the initial constraints for minimization.
This method of calculating equilibrium chemical concentrations is
useful for systems with a large number of different molecules. The use
of
k atomic element conservation equations for the mass
constraint is straightforward, and replaces the use of the
stoichiometric coefficient equations.
[12].
The results are consistent with those specified by chemical equations.
For example, if equilibrium is specified by a single chemical equation:
[16],

where ν
j is the stochiometric coefficient for the
j th molecule (negative for reactants, positive for products) and
Rj is the symbol for the
j th molecule, a properly balanced equation will obey:

Multiplying the first equilibrium condition by ν
j yields

As above, defining ΔG

which will be zero at equilibrium.








![{\displaystyle K_{\mathrm {c} }={\frac {[\mathrm {S} ]^{\sigma }[\mathrm {T} ]^{\tau }}{[\mathrm {A} ]^{\alpha }[\mathrm {B} ]^{\beta }}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1343be135c4f33c7333e53558b9e96d27f1819ed)























![{\displaystyle K={\frac {[\mathrm {S} ]^{\sigma }[\mathrm {T} ]^{\tau }...}{[\mathrm {A} ]^{\alpha }[\mathrm {B} ]^{\beta }...}}\times {\frac {{\gamma _{\mathrm {S} }}^{\sigma }{\gamma _{\mathrm {T} }}^{\tau }...}{{\gamma _{\mathrm {A} }}^{\alpha }{\gamma _{\mathrm {B} }}^{\beta }...}}=K_{\mathrm {c} }\Gamma }](https://wikimedia.org/api/rest_v1/media/math/render/svg/160fbc88403a037161d2c9a44d10cd44746215e8)



![{\displaystyle K_{\mathrm {c} }={\frac {\mathrm {[{CH_{3}CO_{2}}^{-}][{H_{3}O}^{+}]} }{\mathrm {[{CH_{3}CO_{2}H}][{H_{2}O}]} }}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/53b93bf6dae8354f02ab3e935fca63b9bd4a16c4)
![{\displaystyle K={\frac {\mathrm {[{CH_{3}CO_{2}}^{-}][{H_{3}O}^{+}]} }{\mathrm {[{CH_{3}CO_{2}H}]} }}=K_{\mathrm {c} }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/472d349477dcf83e95cb3c3423e8ba247ff1d23a)
![{\displaystyle K_{\mathrm {w} }=\mathrm {[H^{+}][OH^{-}]} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/02e72380cadad70b38e1a6f42ce8b217618bc11f)
![{\displaystyle K_{\mathrm {c} }={\frac {\mathrm {[CO_{2}]} }{\mathrm {[CO]^{2}} }}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/df7ff1e7f45707791cfd9dd8e697fe5695e94f60)
![{\displaystyle {\begin{array}{rl}{\ce {H2A<=>{HA^{-}}+{H+}}}:&K_{1}={\frac {\ce {[HA-][H+]}}{\ce {[H2A]}}}\\{\ce {HA-<=>{A^{2-}}+{H+}}}:&K_{2}={\frac {\ce {[A^{2-}][H+]}}{\ce {[HA-]}}}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/02fc199cec1e474859edf9723bf1aa772591e5a7)

![{\displaystyle \beta _{\ce {D}}={\frac {\ce {[A^{2-}][H^{+}]^{2}}}{\ce {[H_{2}A]}}}=K_{1}K_{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/63732493f38cea73de7cc7600a0fdcac4c2250f9)
![{\displaystyle {\begin{array}{ll}{\ce {{A^{2-}}+{H+}<=>HA-}}:&\beta _{1}={\frac {\ce {[HA^{-}]}}{\ce {[A^{2-}][H+]}}}\\{\ce {{A^{2-}}+{2H+}<=>H2A}}:&\beta _{2}={\frac {\ce {[H2A]}}{\ce {[A^{2-}][H+]^{2}}}}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3bd8050bd8872f9cbd63bffcc07ebc25c6875c3a)


![{\displaystyle T_{\mathrm {A} }=\mathrm {[A]+[HA]+[H_{2}A]} \,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b4caf4ff0552bd0430f4b305b3b92c05aef15aa2)
![{\displaystyle T_{\mathrm {H} }=\mathrm {[H]+[HA]+2[H_{2}A]-[OH]} \,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/094ecf5d3e87ba5a02d744835486f24086804cfc)
![{\displaystyle T_{\mathrm {A} }=\mathrm {[A]} +\beta _{1}\mathrm {[A][H]} +\beta _{2}\mathrm {[A][H]} ^{2}\,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f6ae376d767bfb698f8d771a8aa374366c647255)
![{\displaystyle T_{\mathrm {H} }=\mathrm {[H]} +\beta _{1}\mathrm {[A][H]} +2\beta _{2}\mathrm {[A][H]} ^{2}-K_{w}[\mathrm {H} ]^{-1}\,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/25502da2d24e51d7cb6c53782a5392673c4b0819)
![{\displaystyle T_{\mathrm {A} }=[\mathrm {A} ]+\sum _{i}p_{i}\beta _{i}[\mathrm {A} ]^{p_{i}}[\mathrm {B} ]^{q_{i}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/de4c20306740d1b3f30387f17ac264b500d313b8)
![{\displaystyle T_{\mathrm {B} }=[\mathrm {B} ]+\sum _{i}q_{i}\beta _{i}[\mathrm {A} ]^{p_{i}}[\mathrm {B} ]^{q_{i}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4c9dea321370bf3d5b01364cfed9dbe3c941ce31)









